Winter 2022/2023
The Centers for Medicare & Medicaid Services (CMS) has proposed new rules for 2024 that will address many aspects of prior authorizations in an effort to streamline the process, making them more efficient and effective.
The use of prior authorizations (PA) is currently being evaluated for transparency and effectiveness (see sidebar). This administrative safeguard for appropriate and cost-effective care is often seen by providers and their staff as a burdensome requirement that results in treatment delays.1 However, PA use can be consistent with value-based medicine and is increasingly seen as necessary by payers and pharmacy benefit managers.2 OPN submitted a letter in support of CMS’ efforts to review and potentially change PA rules.
CMS is proposing a number of rules that will affect how PAs are implemented. One key rule is that all Medicare Advantage (MA) plans “establish a Utilization Management (UM) Committee to review policies annually and ensure consistency with traditional Medicare’s national and local coverage decisions and guidelines.”3 This approach can often help to enhance patient outcomes and decrease costs.
Other issues in this proposal that deserve consideration are site of care flexibility (see Spotlight) and that PAs be “based on current widely used treatment guidelines or clinical literature.” Clinical guidelines may not reflect the latest best practices, particularly in oncology. More than 100 new oncology drugs are expected to launch by 2026.* Oncology clinical guidelines unfortunately do not usually keep pace with these new drug launches. Clinical expertise from practicing subspecialists can ensure PA criteria are up-to-date and remain clinically relevant. Reliance solely on current clinical guidance and/or literature may hinder access to new innovative therapies.
* IQIVIA. “The Use of Medicines in the U.S. 2022: Usage and Spending Trends and Outlook to 2026.” Pg. 52. April 2022
Comment Period for the Newly Proposed Rules
In December 2022, CMS proposed rules for many Medicare programs (CMS-4201-P), including significant changes to PA processes. Comments were due by February 13, 2023. These rules were meant to update and clarify regulations regarding coverage criteria and Utilization Management policies for MA plans. It is intended to ensure that MA enrollees receive the same access to medically necessary care as traditional Medicare enrollees. In addition, it proposes policies to streamline PA requirements and reduce disruption for MA enrollees.
Important Links for New CMS Proposal
Proposed Rules • Press Release • Fact Sheet
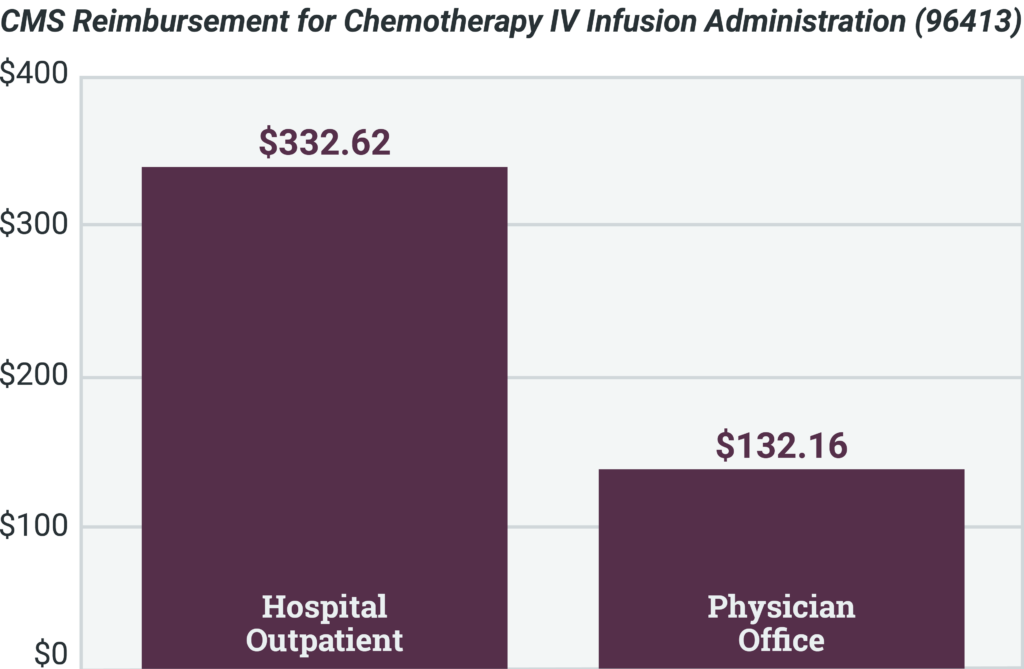
Spotlight: Site of Care Impact
Site-of-care optimization programs are effective for providing patients access to the most cost- effective care in the most cost-effective setting. Shifting to specific settings reduces costs for patients and payers, providing savings to the system. Some reimbursements for hospitals can be more than double the cost of the same procedure being administered in a physician’s office.
For further information, please contact:
Adam Goldston (email)
Chief Growth Officer
OPN Healthcare