Understanding how to evaluate chemotherapy and radiation oncology regimens
The Oncology Physicians Network (OPN) Care Management Services program offers a sophisticated approach to oncology care through data driven technology and advanced clinical expertise. The program utilizes a team of medical oncology subspecialists and clinical pharmacists who work with participating oncologists to provide optimum care for patients. This care is based on national practice guidelines such as the National Comprehensive Cancer Network (NCCN) Guidelines for oncology, an important resource in assisting physicians and pharmacists to determine the best evidence-based treatment and improve the quality of cancer care. However, some treatments are not always appropriate due to associated toxicities, ineffective therapies or invasive procedures with associated risks. The following case studies illustrate inappropriate therapies being considered and why such regimens are not advisable for clinical reasons. Additionally, such regimens have an associated cost that would financially impact the covering risk bearing organizations (RBOs) and the patients. In all cases, patient care is at the forefront of what is being assessed.
Pulmonary Toxicities and Brentuximab
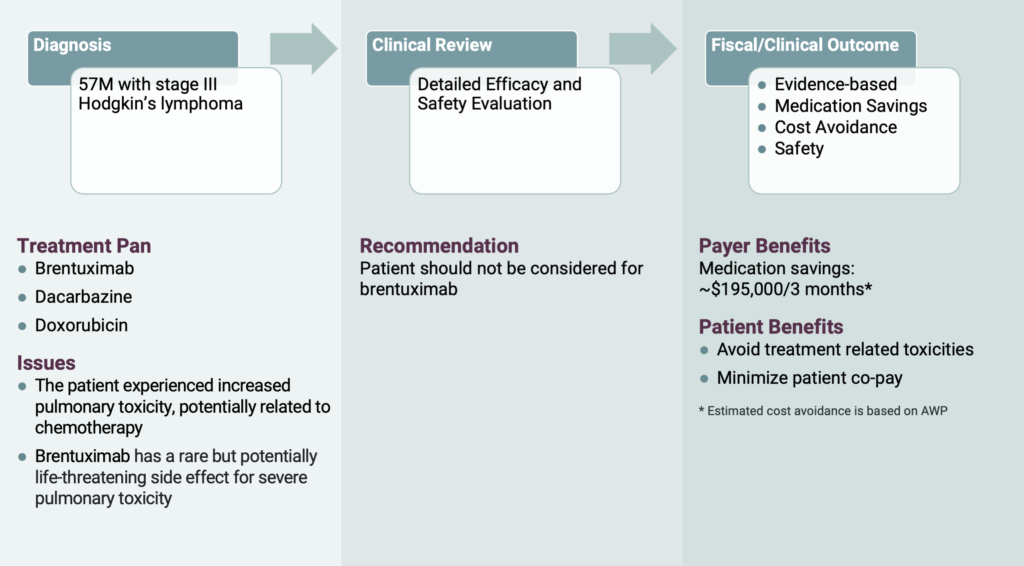
Take the recent case of a 57-year-old male patient with stage III Hodgkin’s Lymphoma. His oncologist requested brentuximab, dacarbazine and doxorubicin to help treat his Hodgkin’s Lymphoma. However, OPN’s clinical pharmacist noticed the patient had increased pulmonary toxicity, a damage to the lungs, possibly due to the chemotherapy the patient was receiving. Brentuximab has a rare, but potentially life-threatening side effect for severe pulmonary toxicity, which could potentially cause more damage to the patient’s lungs if administered. There are other regimens for use in this setting such as BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine and procarbazine) and ABVD (doxorubicin, bleomycin, vinblastine and dacarbazine). However, because bleomycin also has the major limitation of pulmonary toxicity, OPN felt it was appropriate to intervene and hold brentuximab and continue with dacarbazine and doxorubicin until the physician deemed appropriate to continue with brentuximab. As a result of this intervention, treatment related toxicities were avoided, and the patient’s co-pay was minimized. In addition, OPN helped save the covering RBO approximately $200,000 for the authorization duration of 3 months.
Using Azacitidine as a Frontline Treatment
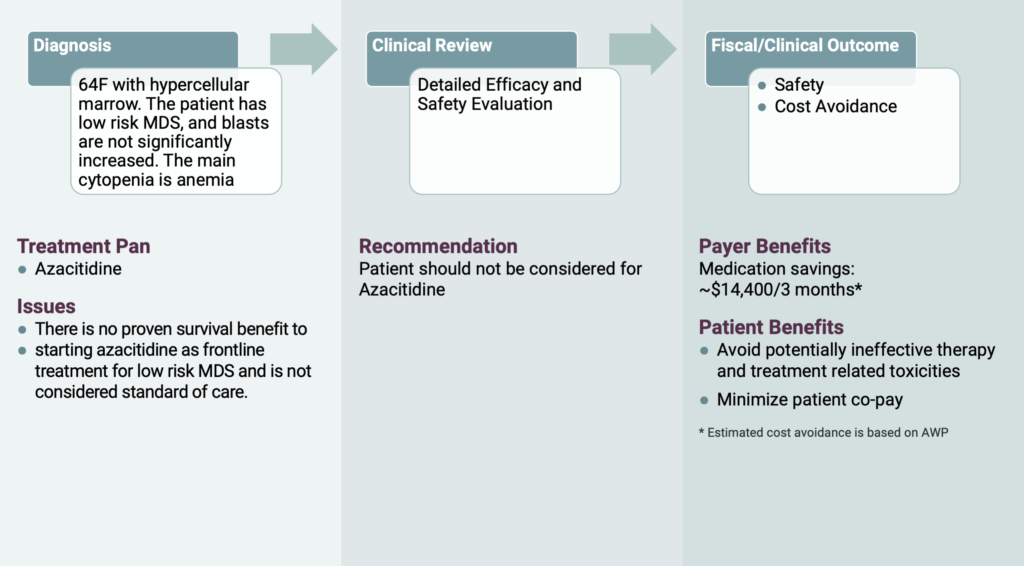
Now let’s consider a 64-year-old female patient with hypercellular marrow. The patient had low risk myelodysplastic syndrome (MDS) with the main cytopenia being anemia. Her oncologist requested for azacitidine. However, OPN’s oncology subspecialist recommended the patient have her erythropoietin checked. If the results were less than 500 mU/mL, the patient would be eligible to receive treatment with erythropoietin stimulating agent (ESA). If the patient failed to respond to ESA or progressed on ESA, a granulocyte colony-stimulating factor (GCSF) would be beneficial to add to the ESA for increased efficacy. If the treating physician is concerned for elevated risk disease features, a next generation sequencing can be done on the blood or bone marrow to rule out high risk molecular abnormalities that carry a greater possibility for transformation to AML. Because there is no survival benefit to starting azacitidine as a frontline treatment for low risk MDS and it is not considered standard of care, OPN felt it is important to intervene on this medication. As a result of this intervention, treatment related toxicities were avoided, and the patient’s co-pay was minimized. In addition, OPN helped save the covering RBO approximately $14,000 for the authorization duration of 3 months.
Improper Use of Y-90 Resin for Thyroid Cancer
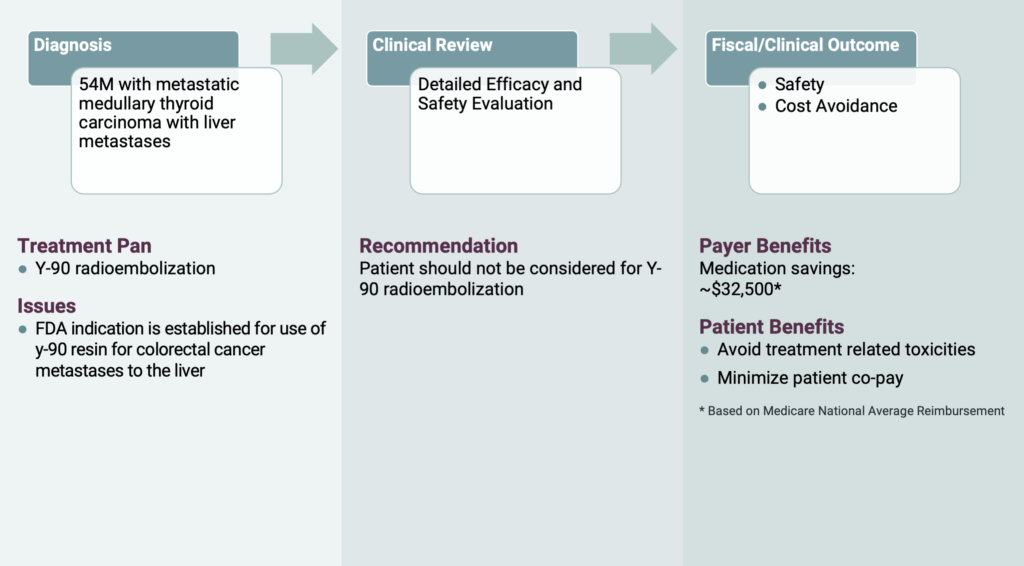
The last case is related to a 54-year-old male with metastatic medullary thyroid cancer. His radiation oncologist requested Y-90 radioembolization, which combines embolization and radiation to treat liver cancer. However, OPN’s radiation oncologist noted that FDA approval for Y-90 resin is for colorectal cancer that has metastasized to the liver. The patient did not meet these criteria since the primary tumor was thyroid cancer. Such an invasive procedure and its associated risks was not warranted and there was no clinically proven benefit to such a regimen. OPN helped the RBO save over $30,000 and reduced the patients out of pocket costs.
Considerations
OPN understands that any regimen intervention is part of a collaborative process between the Care Management team and the referring providers. All regimen reviews are subject to follow-up considerations. If a provider feels that the intervention was not accurately assessed, they can request a peer-to-peer discussion to review the care being evaluated. In a recent month this happened less than 7% of the time with fewer than 2% of the interventions being overturned. In all cases the Care Management team works to follow applicable guidelines and provide clinical guidance that takes into account unnecessary toxicity, overutilization and the patient benefit of any therapy being considered.